Artificial Serum
Complement for Serum Free Medium
Product Basics
A complete serum free cell culture system is beneficial to eliminate or reduce unknown contaminants and reduce lot to lot variation. It provides consistency in culturing healthy cells, in turn maintaining consistency in your results. This Artificial Serum is developed by Cell Science and Technology Institute to complement their serum free medium, such as HFDM-1 and ALyS series so that researchers can achieve a complete serum free cell culture system. This Artificial Serum requires only 20-50% of the concentration of conventional serum (e.g., FBS), and is available in Xeno-free or Animal-free.
Key Features
- Serum Free
- Available in Xeno-free or Animal-free
- Suitable for culturing human stem cells, primary cells, and cell lines with both adherent and non-adherent cell culture systems
- Verified cells: Fibroblast, Mesenchymal stem cells, Vero cells, PBMC, and DAUDI cells
- Certified by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, to be safe to use in regenerative medicine
- Manufactured in Compliance with JPN GMP guidelines
Technical Information
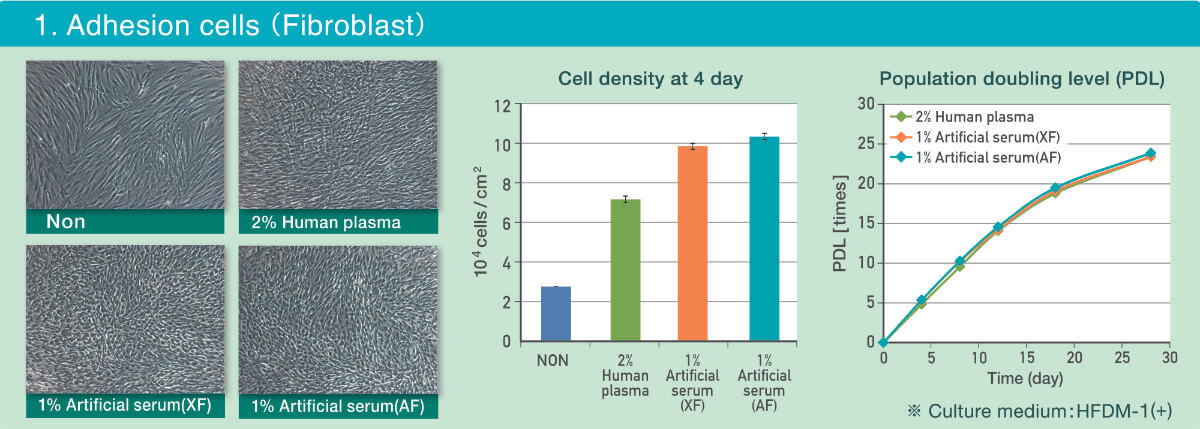
The below figures are a comparison of Normal Adult Human Dermal Fibroblast (Lonza) cultured in HFDM-1™ (+) (CSTI Part no. 2102P05) supplemented with human plasma (2%) and the Artificial Serum (1%).
- Morphology and cell proliferation cultured in both xeno-free and animal free Artificial Serum were equivalent to the cells cultured with human plasma (images taken at day 4).
- Cell density cultured with was higher with the Artificial Serum compared to human plasma at day 4.
- Population doubling level (PDL) was similar in both 2% human plasma and 1% Artificial Serum, 30 days of culture.
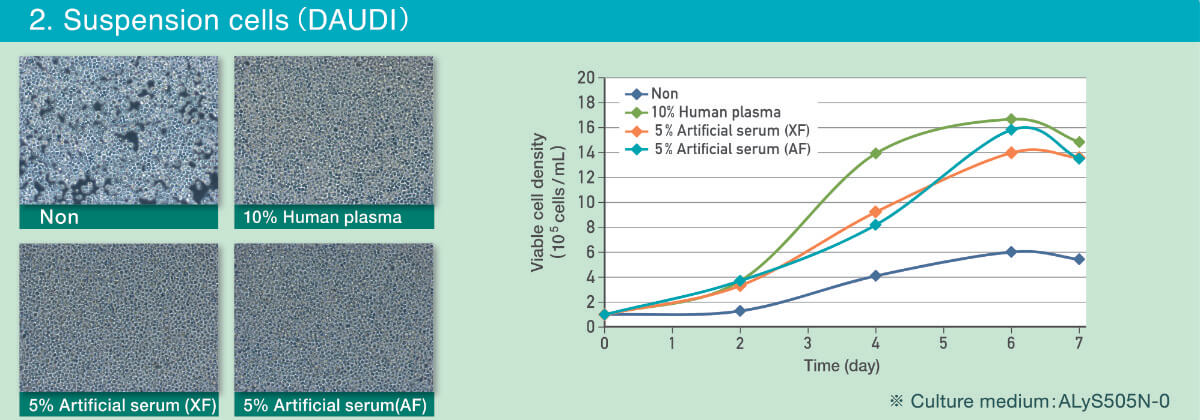
Suspension culture system (DAUDI cells)
The below figures are a comparison of DAUDI cells cultured in ALyS™505N-0 (CSTI Part No. 1020P10) supplemented with human plasma (10%) and the Artificial Serum (5%).
- Morphology and cell proliferation cultured in both xeno-free and animal free Artificial Serum were equivalent to the cells cultured with human plasma (images taken at day 4).
- Culturing with 10% human plasma showed higher viable cell density (VCD) at the beginning, but by day 6 the Artificial Serum had reached a similar VCD.
Specification
- Available in Xeno-free or Animal-free
- Size: 20ml
- Storage temperature: -20°C
- Shelf life: 18 months following manufacture date
- Manufactured by: Cell Science and Technology Institute
Pricing
Xeno Free
- SKU: A2G10P2CC
- Size: 20ml
- Price:
$500.00→ $450.00
Animal Free
- SKU: A2G20P2CC
- Size: 20ml
- Price:
$850.00→ $765.00
References
- Mitsuno, K. et al. Selective JAK2 pathway inhibition enhances anti-leukemic functionality in CD19 CAR-T cells. Cancer Immunology, Immunotherapy 74, 79 (2025) doi: 1007/s00262-024-03927-8.
- Suematsu, M. et al. Targeting FLT3-specific chimeric antigen receptor T cells for acute lymphoblastic leukemia with KMT2A rearrangement. Cancer Immunology, Immunotherapy 72, 957–968 (2023) doi: 1007/s00262-022-03303-4.
- Chinsuwan, T. et al. Ligand-based, piggyBac-engineered CAR-T cells targeting EGFR are safe and effective against non-small cell lung cancers. Molecular Therapy – Oncolytics 31, (2023) doi: 1016/j.omto.2023.100728.
- Suematsu, M. et al. PiggyBac Transposon-Mediated CD19 Chimeric Antigen Receptor-T Cells Derived From CD45RA-Positive Peripheral Blood Mononuclear Cells Possess Potent and Sustained Antileukemic Function. Frontiers in Immunology 13, (2022) doi: org/10.3389/fimmu.2022.770132.
- Hiramatsu, N. et al. Formation of three‑dimensional cell aggregates expressing lens‑specific proteins in various cultures of human iris‑derived tissue cells and iPS cells. Exp Ther Med 24, 539 (2022) doi: 3892/etm.2022.11476.
- Yamamoto, N. et al. Novel Technique for Retinal Nerve Cell Regeneration with Electrophysiological Functions Using Human Iris-Derived iPS Cells. Cells 10, (2021) doi: 3390/cells10040743.
- Yagyu, S. et al. A lymphodepleted non-human primate model for the assessment of acute on-target and off-tumor toxicity of human chimeric antigen receptor-T cells. Clinical & Translational Immunology 10, e1291 (2021) doi: 1002/cti2.1291.
- Tomida, A. et al. Inhibition of MEK pathway enhances the antitumor efficacy of chimeric antigen receptor T cells against neuroblastoma. Cancer Sci 112, 4026–4036 (2021) doi: 1111/cas.15074.
- Nakamura, K. et al. Autologous antigen-presenting cells efficiently expand piggyBac transposon CAR-T cells with predominant memory phenotype. Molecular Therapy – Methods & Clinical Development 21, 315–324 (2021) doi: 1016/j.omtm.2021.03.011.
- Kubo, H. et al. Development of non-viral, ligand-dependent, EPHB4-specific chimeric antigen receptor T cells for treatment of rhabdomyosarcoma. Molecular Therapy – Oncolytics 20, 646–658 (2021) doi: 1016/j.omto.2021.03.001.
- Morokawa, H. et al. Autologous non-human primate model for safety assessment of piggyBac transposon-mediated chimeric antigen receptor T cells on granulocyte–macrophage colony-stimulating factor receptor. Clinical & Translational Immunology 9, e1207 (2020) doi: 1002/cti2.1207.
Other Documents
- Protocol - Coming soon
- SDS (Xeno-free)
- SDS (Animal-free)
- Sample CoA (Xeno-free)
- Sample CoA (Aminal-free)
FOR RESEARCH USE ONLY, NOT FOR USE IN DIAGNOSTIC PROCEDURES