iMatrix 511 Series
Maintenance and expansion of pluripotent stem cells (PSCs) & mesenchymal stem cells (MSCs)
Product Basics
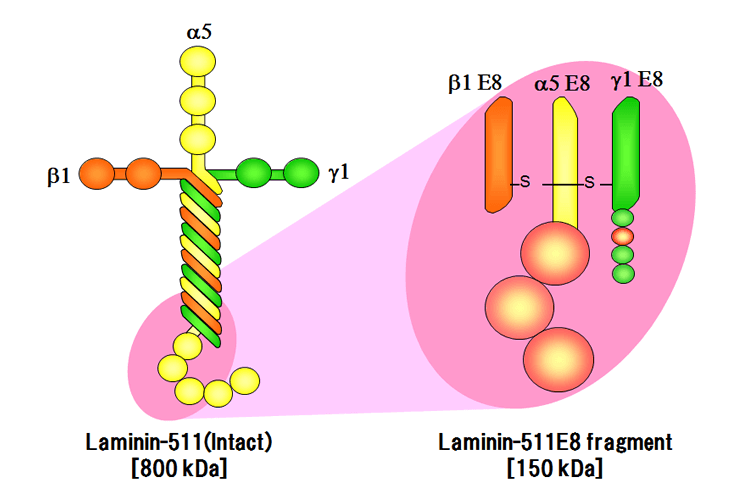
The iMatrix-511 is an innovative cell culture matrix that is compatible with a wide variety of cell types. It is exceptionally well-suited for pluripotent and mesenchymal stem cells. This product consists of recombinant Laminin-511 E8 protein fragments, which allow ES/iPS cells and MSCs to be maintained in xeno-free culture conditions. It also enables the passaging of single cells and provides greater adhesion than full-length Laminin, Vitronectin, or Matrigel (Miyazaki et al., Nature Commun. 3; 1236 (2012)).
Key Features
- Ideal for Xeno-Free, Animal-Free, Feeder-Free Cell Culture
- Proven to provide superior adhesion of human ES and iPS cells¹
- Enables the passaging of single cells
- Compatible with a wide variety of cell types
- Facilitates the extended culture of hES/hiPS cells and hMSCs
- No pre-coating required
Technical Information
Greater Cell Adhesion & Proliferation
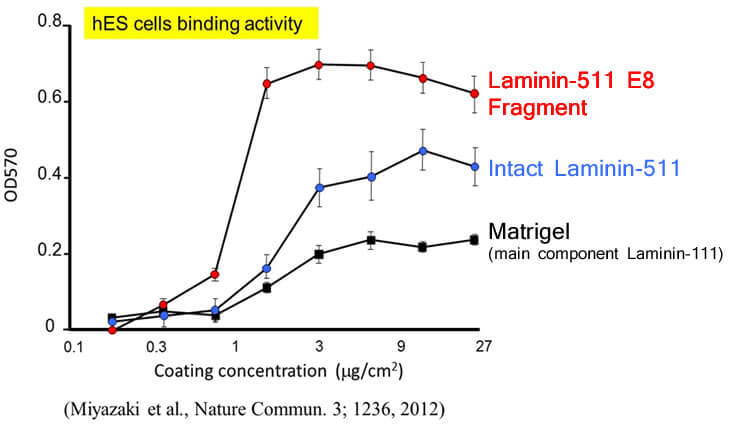
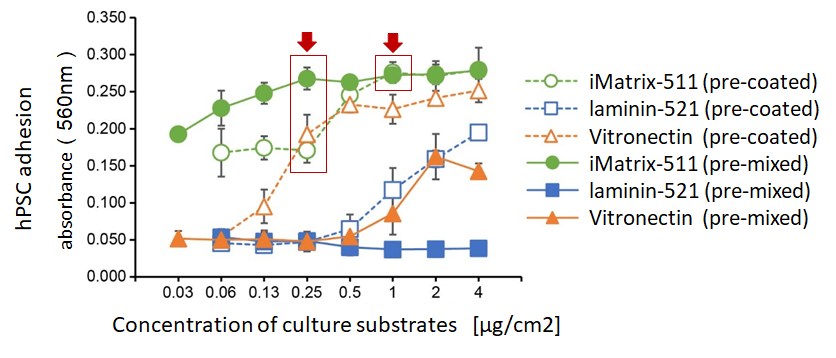
In the referenced study above, the adhesion strength of the Laminin-511 E8 fragment and other cell culture substrates to hES cells were compared. The horizontal axis of the graph represents the concentration of the cell culture substrate, while the vertical axis represents the OD value (optical density at 570nm). These results demonstrate that the Laminin-511 E8 fragment adheres to cells more strongly than its competitors.
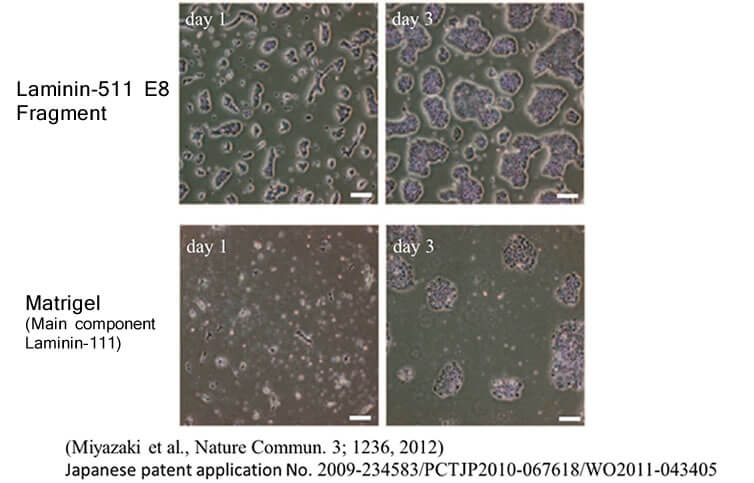
In the same study mentioned above, human ES cells were passaged on Laminin-511 E8 fragment and other cell culture substrates. When human ES cell colonies were dispersed individually at the time of passage, it was observed that single cells adhered rapidly to Laminin-511 E8 and proliferated immediately.
Maintain and Culture Single ES/iPS Cells with Greater Propagation
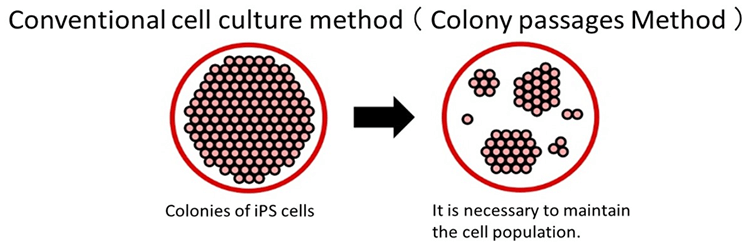
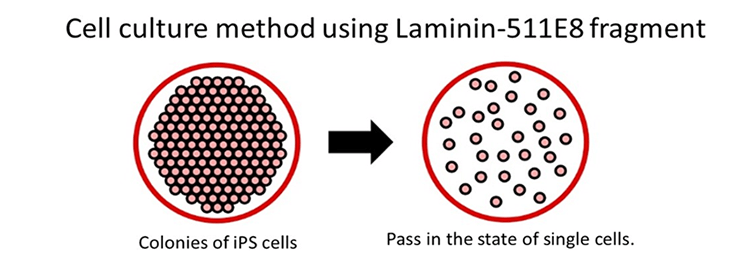
With conventional methods, ES/iPS cells perish when dispersed into single cells, necessitating the maintenance of a cell colony during passaging.
Using the Laminin-511 E8 fragment as a cell culture matrix enables the culturing of single ES/iPS cells, which in turn reduces the time required to optimize culture methods and drastically enhances the efficiency of cell cultures.
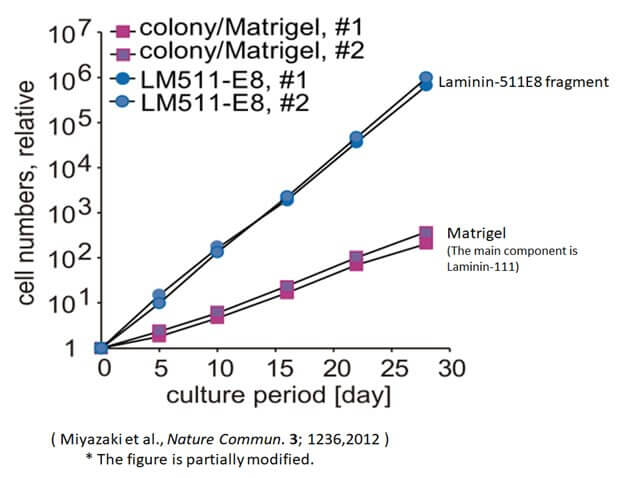
Culture more ES/iPS cells using Laminin-511 E8 fragment
The graph above compares the numbers of ES/iPS cells cultured by the conventional method (colony) for 30 days with those cultured using the Laminin-511 E8 fragment. The results confirm that the cell count increased by at least 200 times when the Laminin-511 E8 fragment was employed.
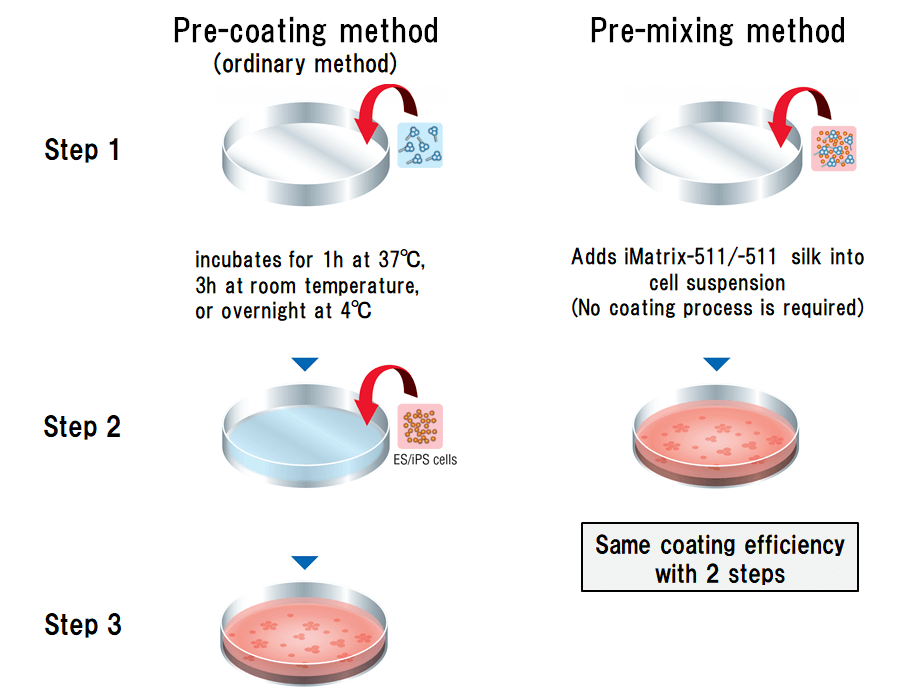
No pre-coating required
- Reduces preparation time.
- Reduces the amount to use by about half compared to pre-coating method.
Reduces the amount to use by about half compared to pre-coating method.
The pre-mixed method reduces the amount of substrate needed for maximum adhesion of hPSCs compared to pre-coat method.
Specification
- Format: Liquid Solution (175 ug/vial @0.5 mg/mL), Solvent: PBS (-)
- Storage temperature: 2-15 °C
- Manufactured by : Nippi, Inc. 1-1-1 Senju-Midoricho, Adachi-ku, Tokyo, Japan
Pricing
iMatrix-511
- Expressed by CHO-S Cell
- Liquid Solution (175 ug/vial @0.5 mg/mL), Solvent: PBS (-)
- 175ug coats roughly 2-6 six well plates via conventional method
- SKU: N-892013
- Price: $80.00
- Incl. US (Continental) shipping fee
- * Only one purchase per lab *
350ug size (175ug x 2 tubes)
- SKU: N-892011
- Price:
$270.00→ $216.00
1050ug size (175ug x 6 tubes)
- SKU: N-892012
- Price:
$690.00→ $570.00
iMatrix-511 Silk
- Expressed by transgenic silkworm cocoon
- Cost-efficient alternative of iMatrix-511
- Liquid Solution (175 ug/vial @0.5 mg/mL), Solvent: PBS (-)
- 175ug coats roughly 2-6 six well plates via conventional method
- Similar spec as standard iMatrix-511 but more cost effective
- SKU: N-892022
- Price: $80.00
- Incl. US (Continental) shipping fee
- * Only one purchase per lab *
Easy iMatrix-511
- Pre-diluted at a concentration of 1.6ug/ml
- Ready to Use
- 100mL = roughly 16 6-well plate (amount to coat well surface = 0.25ug/cm²)
Easy iMatrix-511 (CHO-S Cell)
- SKU: N-892019
- Price: $80.00
- Incl. US (Continental) shipping fee
- * Only one purchase per lab *
- SKU: N-892018
- Price: $350.00
Easy iMatrix-511 Silk (Transgenic silkworm cocoon)
- SKU: N-892025
- Price: $80.00
- Incl. US (Continental) shipping fee
- * Only one purchase per lab *
- SKU: N-892024
- Price: $305.00
References & Literature
Ayabe, H., Anada, T., Kamoya, T., Sato, T., Kimura, M., Yoshizawa, E., Kikuchi, Shunyuu., Ueno, Yasuharu., Sekine, keisuke., J. Gray Camp., Treutlein, B., Ferguson, Autumn., Suzuki, Osamu., Takede, Takanori.. Optimal Hypoxia Regulates Human iPSC-Derived Liver Bud Differentiation through Intercellular TGFB Signaling. Stem Cell Reports, 11, 1-11, (2018)
Musah, S., Dimitrakakis, N., Camacho, D. M., Church, G. M., Ingber, D. E.. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a lomerulus Chip. Nature protocols, 13(7), 1662, (2018)
Ishii, K., Sakurai, H., Suzuki, N., Mabuchi, Y., Sekiya, I., Sekiguchi, K., Akazawa, C.. Recapitulation of Extracellular LAMININ Environment Maintains Stemness of Satellite Cells In Vitro. Stem Cell Reports, 10, 1-15, (2018)
Ishida, K., Xu, H., Sasakawa, N., Lung, M. S. Y., Kudryashev, J. A., Gee, P., & Hotta, A.. Site-specific randomization of the endogenous genome by a regulatable CRISPR-Cas9 piggyBac system in human cells. Scientific Reports, 8(1), 310, (2018)
Takayama, K., Hagihara, Y., Toba, Y., Sekiguchi, K., Sakurai, F., Mizuguchi, H.. Enrichment of high-functioning human iPS cell-derived hepatocyte-like cells for pharmaceutical research. Biomaterials, (2018)
Akiyama, T., Sato, S., Chikazawa-Nohtomi, N., Soma, A., Kimura, H., Wakabayashi, S., Ko, S. B., Ko, M. S.. Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing. Scientific Reports, 8(1), 1189, (2018)
Saito, A., Ooki, A., Nakamura, T., Onodera, S., Hayashi, K., Hasegawa, D., Okudaira,T., Watanabe, K., Kato, H., Onda, T., Watanabe, A., Kosaki, K., Nishimura, K., Ohtaka, Manami., Nakanishi, M., Sakamoto, T., Yamaguchi, A., Sueishi, K., Azuma, T.. Targeted reversion of induced pluripotent stem cells from patients with human cleidocranial dysplasia improves bone regeneration in a rat calvarial bone defect model. Stem Cell Research & Therapy, 9(1), 12, (2018)
Yamauchi, K., Li, J., Morikawa, K., Liu, L., Shirayoshi, Y., Nakatsuji, N., Elliott, A. D., Hisatome, I., Suemori, H..Isolation and characterization of ventricular-like cells derived from NKX2-5 eGFP/w and MLC2v mCherry/w double knock-in human pluripotent stem cells. Biochemical and Biophysical Research Communications, 495(1), 1278-1284, (2018)
Mae, S., Ryosaka, M., Toyoda, T., Matsuse, K., Oshima, Y., Tsujimoto, H., Okumura, S., Shibasaki, A., Osafune, K.. Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochemical and biophysical research communications, 495(1), 954-961, (2018)
Kagihiro, M., Fukumori, K., Aoki, T., Ungkulpasvich, U., Mizutani, M., Viravaidya-Pasuwat, K., & Kino-oka, M.. Kinetic analysis of cell decay during the filling process: Application to lot size determination in manufacturing systems for human induced pluripotent and mesenchymal stem cells. Biochemical Engineering Journal, 131, 31-38, (2018)
Zhang, R. R., Koido, M., Tadokoro, T., Ouchi, R., Matsuno, T., Ueno, Y., Sekine, K., Takebe, T., Taniguchi, H.. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Reports, 10(2), 1?14, (2018)
Oshima, K., Saiki, N., Tanaka, M., Imamura, H., Niwa, A., Tanimura, A., Nagahashi, A., Hirayama, A., Okitac, K., Hotta, A., Kitayama, S., Osawa, M., Kaneko, S., Watanabe, A., Asaka, I., Fujibuchi, W., Imai, K., Yabe, H., Kamachi, Y., Hara, J., Kojima, S., Tomita, M., Soga, T., Noma, T., Nonoyama, S., Nakahata, T., Saito, MK.. Human AK2 links intracellular bioenergetic redistribution to the fate of hematopoietic progenitors. Biochemical and Biophysical Research Communications, 497(2), 719-725, (2018)
Sougawa, N., Miyagawa, S., Fukushima, S., Kawamura, A., Yokoyama, J., Ito, E., Harada, A., Okimoto, K., Mochisuki-Oda, N., Saito, A., Sawa, Y.. Immunologic targeting of CD30 eliminates tumourigenic human pluripotent stem cells, allowing safer clinical application of hiPSC-based cell therapy. Scientific Reports, 8(1), 3726, (2018)
Yasuda, S. Y., Ikeda, T., Shahsavarani, H., Yoshida, N., Nayer, B., Hino, M., Vartak-Sharma, N.,Suemori, H., Hasegawa, K.. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nature Biomedical Engineering, 2(3), 173, (2018)
Kim, S. I., Matsumoto, T., Kagawa, H., Nakamura, M., Hirohata, R., Ueno, A., Ohishi, M., Sakuma, T., Soga, T., Yamamoto, T., Woltjen, K.. Microhomology-assisted scarless genome editing in human iPSCs. Nature Communications, 9(1), 939, (2018)
Li, L., Roh, J. H., Chang, E. H., Lee, Y., Lee, S., Kim, M., Koh, W., Chang, J. W., Kim, H. J., Nakanishi, M., Barker, R. A., Na, D. L., Song, J.. iPSC Modeling of Presenilin1 Mutation in Alzheimer’s Disease with Cerebellar Ataxia. Experimental Neurobiology, 27, 27(5), 350-364, (2018)
Hayashi, R., Ishikawa, Y., Katori, R., Sasamoto, Y., Taniwaki, Y., Takayanagi, Tsujikawa, M., Sekiguchi, K., Quantock, A. J., Nishida, K. . Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nature Protocols, 12(4), 683-696, (2017)
Kikuchi, T., Morizane, A., Okita, K., Nakagawa, M., Yamakado, H., Inoue, H., Takahashi, R., Takahashi, J. . Idiopathic Parkinson’s disease patient‐derived induced pluripotent stem cells function as midbrain dopaminergic neurons in rodent brains. Journal of Neuroscience Research, 95(9),1829-37, (2017)
Miyazaki, T., Isobe, T., Nakatsuji, N., & Suemori, H. . Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner. Scientific Reports, 7(41165), 1-8, (2017)
Goparaju, S. K., Kohda, K., Ibata, K., Soma, A., Nakatake, Y., Akiyama, T., Wakabayashi, S., Matsushita, M., Sakota, M., Kimura, H., Yuzaki, M., Shigeru B. H. Ko & Minoru S. H. Ko. . Rapid differentiation of human pluripotent stem cells into functional neurons by mRNAs encoding transcription factors. Scientific Reports, 7, 42367, (2017)
Musah, S., Mammoto, A., Ferrante, C. T., Jeanty, S.S., Hirano-Kobayashi, M., Mammoto, T., Roberts, K., Chung, S., Novak, R., Ingram, M., Fatanat-Didar, T., Koshy, S., Weaver, C. J., Church, M. G., Ingber, F. D. . Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nature Biomedical Engineering, 1 (0069), (2017)
Camp, J. G., Sekine, K., Gerber, T., Loeffler-Wirth, H., Binder, H., Gac, M., Kanton, S., Kageyama, J., Damm, G., Seehofer, D., Belicova, L., Barsacchi, M., Barsacchi, R., Okuda, R., Yoshizawa, E., Kimura, M., Ayabe, H., Taniguchi, H., Takebe, T., & Belicova, L.. Multilineage communication regulates human liver bud development from pluripotency. Nature, 546, 533-538, (2017)
Polisetti, N., Sorokin, L., Okumura, N., Koizumi, N., Kinoshita, S., Kruse, F. E., and Schlotzer-Schrehardt, U. Laminin-511 and-521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Scientific Reports, 7, 5152, (2017)
Hongo, A., Okumura, N., Nakahara, M., Kay, E. P., & Koizumi, N.. The Effect of a p38 Mitogen-Activated Protein Kinase Inhibitor on Cellular Senescence of Cultivated Human Corneal Endothelial CellsEffect of a p38 MAPK Inhibitor on Corneal Endothelial Cells. Investigative Ophthalmology & Visual Science, 58(9), 3325-3334, (2017)
Taniguchi, Y., Li, S., Takizawa, M., Oonishi, E., Toga, J., Yagi, E., & Sekiguchi, K. Probing the acidic residue within the integrin binding site of laminin-511 that interacts with the metal ion-dependent adhesion site of α6β1 integrin. Biochemical and Biophysical Research Communications, 487(3), 525-531, (2017)
Sekine, S. I., Kondo, T., Murakami, N., Imamura, K., Enami, T., Shibukawa, R., Tsukita, K., Funayama, M., Inden, M., Kurita, H., Hozumi, I., Inoue, H.. Induced pluripotent stem cells derived from a patient with familial idiopathic basal ganglia calcification (IBGC) caused by a variant in SLC20A2 gene. Stem Cell Research, (2017)
Tan, G. W., Kondo, T., Murakami, N., Imamura, K., Enami, T., Tsukita, K., Shibukawa, R., Funayama, M., Matsumoto, R., Ikeda, I., Takahashi, R., Inoue, H.. Induced pluripotent stem cells derived from an autosomal dominant lateral temporal epilepsy (ADLTE) patient carrying S473L mutation in leucine-rich glioma inactivated 1 (LGI1). Stem Cell Research, (2017)
Sato-Nishiuchi, R., Li, S., Ebisu, F., Sekiguchi, K.. Recombinant laminin fragments endowed with collagen-binding activity: A tool for conferring laminin-like cell-adhesive activity to collagen matrices. Matrix Biology, (2017)
Kikuchi, T., Morizane, A., Doi, D., Magotani, H., Onoe, H., Hayashi, T., Mizuma, H., Takara, S., Takahashi, R., Inoue, H., Morita, S., Yamamoto, M., Okita, K., Nakagawa, M., Parmar, M., Takahashi, J.. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature, 548, 592-596, (2017)
Takizawa, M., Arimori, T., Taniguchi, Y., Kitago, Y., Yamashita, E., Takagi, J., Sekiguchi, K.. Mechanistic basis for the recognition of laminin-511 by α6β1 integrin. Science Advances, 3(9), e1701497, (2017)
Morizane, A., Kikuchi, T., Hayashi, T., Mizuma, H., Takara, S., Doi, H., Mawatari, A., Glasser, M. F., Shiina, T., Ishigaki, H., Itoh, Y., Okita, K., Yamasaki, E., Doi, D., Onoe, H., Ogasawara, K., Yamanaka, S., and Takahashi, J. . MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nature Communications, 8(1), 385, (2017)
Kikuchi, T., Morizane, A., Doi, D., Magotani, H., Onoe, H., Hayashi, T., Mizuma, H., Takara, S., Takahashi, R., Inoue, H., Morita, S., Yamamoto, M., Okita, K., Nakagawa, M., Parmar, M., Takahashi, J. . human ips cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature, 548(7669), 592-596, (2017)
Kojima, Y., Sasaki, K., Yokobayashi, S., Sakai, Y., Nakamura, T., Yabuta, Y., Nakaki, F., Nagaoka, S., Woltjen, K., Hotta, A., Yamamoto, T., Saitou, M.. Evolutionarily Distinctive Transcriptional and Signaling Programs Drive Human Germ Cell Lineage Specification from Pluripotent Stem Cells. Cell Stem Cell, 21(4), 517-532.e5, (2017)
Taguchi, A., & Nishinakamura, R.. Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell, 21. (2017)
Takebe, T., Sekine, K., Kimura, M., Yoshizawa, E., Ayano, S., Koido, M., Funayama, S., Nakanishi, N., Hisai, T., Kobayashi, T., Kasai, T., Kitada, R., Mori, A., Ayabe, H., Ejiri, Y., Amimoto, N., Yamazaki, Y., Ogawa, S., Ishikawa, M., Kiyota, Y., Sato, Y., Nozawa, K., Okamoto, S., Ueno, Y., Kasai, T.. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Reports, 21(10), 2661-2670, (2017)
Uchimura, T., Otomo, J., Sato, M., Sakurai, H.. A human iPS cell myogenic differentiation system permitting high-throughput drug screening. Stem cell research, 25, 98-106, (2017)
Sougawa, N., Miyagawa, S., Fukushima, S., Saito, A., Yokoyama, J., Kitahara, M., Harada, A., Sato-Nishiuchi, R., Sekiguchi, K., Sawa, Y.. Novel Stem Cell Niches Laminin 511 Promotes Functional Angiogenesis Through Enhanced Stem Cell Homing by Modulating” Stem Cell Beds” in the Failed Heart.Circulation, 136(1), A15587, (2017)
Samata, B., Doi, D., Nishimura, K., Kikuchi, T., Watanabe, A., Sakamoto, Y., Kakuta, J., Ono, Y., & Takahashi, J.. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nature Communications, 7(13097), 1-11, (2016)
Hayashi, R., Ishikawa, Y., Sasamoto, Y., Katori, R., Nomura, N., Ichikawa, T., Araki, S., Soma, T., Kawasaki, S., Sekiguchi, K., Tsujikawa, M., Nishida, K., & Quantock, A. J.. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature, 531(7594), 376-380, (2016)
Matsuno, K., Mae, S. I., Okada, C., Nakamura, M., Watanabe, A., Toyoda, T., Uchida, E., Osafune, K.. Redefining definitive endoderm subtypes by robust induction of human induced pluripotent stem cells. Differentiation; research in biological diversity, (2016)
Nishimura, K., Doi, D., Samata, B., Murayama, S., Tahara, T., Onoe, H., & Takahashi, J.. Estradiol Facilitates Functional Integration of iPSC-Derived Dopaminergic Neurons into Striatal Neuronal Circuits via Activation of Integrin α5β1. Stem cell reports, 6(4), 511-524, (2016)
Takayama, K., Mitani, S., Nagamoto, Y., Sakurai, F., Tachibana, M., Taniguchi, Y., Sekiguchi, K., Mizuguchi, H.. Laminin 411 and 511 promote the cholangiocyte differentiation of human induced pluripotent stem cells. Biochemical and biophysical research communications, 474(1), 91-96, (2016)
Kawamura, T., Miyagawa, S., Fukushima, S., Maeda, A., Kashiyama, N., Kawamura, A., Miki, K., Okita, K., Yoshida, Y., Shiina, T., Ogasawara, K., Miyagawa, S., Toda, K., Okuyama, H., Sawa, Y.. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem cell reports, 6(3), 312-320, (2016).
Tanigawa, S., Taguchi, A., Sharma, N., Perantoni, A. O., & Nishinakamura, R.. Selective in vitro propagation of nephron progenitors derived from embryos and pluripotent stem cells. Cell reports, 15(4), 801-813, (2016)
Okumura, N., Kakutani, K., Numata, R., Nakahara, M., Schlotzer-Schrehardt, U., Kruse, F., Kinoshita. K., Koizumi, N.. Laminin-511 and-521 Enable Efficient In Vitro Expansion of Human Corneal Endothelial CellsLaminin-511 and-521 Enable Expansion of HCECs. Investigative ophthalmology & visual science, 56(5), 2933-2942, (2015)
Sasaki, K., Yokobayashi, S., Nakamura, T., Okamoto, I., Yabuta, Y., Kurimoto, K., Ohta, H., Moritoki, Y., Iwatani, C., Tsuchiya, H., Nakamura, S., Sekiguchi, K., Sakuma, T., Yamamoto, T., Mori, T., Woltjen, K., Nakagawa, M., Yamamoto, T., Takahashi, K., Yamanaka, S., Saitou, M.. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell stem cell, 17(2), 178-194, (2015)
Nakagawa, M., Taniguchi, Y., Senda, S., Takizawa, N., Ichisaka, T., Asano, K., Morizane, A., Doi, D., Takahashi, J., Nishizawa, M., Yoshida, Y., Toyoda, T., Osafune, K., Sekiguchi, K., & Yamanaka, S. . A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Scientific reports, 4(3594), 1-7, (2014)
Doi, D., Samata, B., Katsukawa, M., Kikuchi, T., Morizane, A., Ono, Y., Sekiguchi, K., Nakagawa, M., Parmar, M., Takahashi, J.. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem cell reports, 2(3), 337-350, (2014)
Takashima, Y., Guo, G., Loos, R., Nichols, J., Ficz, G., Krueger, F., Oxley, D., Santos, F., Clarke, J., Mansfield, W., Reik, W., Bertone, P., Smith, A.. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell, 158(6), 1254-1269, (2014)
Fukuta, M., Nakai, Y., Kirino, K., Nakagawa, M., Sekiguchi, K., Nagata, S., Matsumoto, Y., Yamamoto, T., Umeda, K., Heike, T., Okumura, N., Koizumi, N., Sato, T., Nakahata, T., Saito, M., Otsuka, T., Kinoshita, S., Ueno, M., Ikeya, M., Toguchida, J. . Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PloS one, 9(12), e112291, (2014)
Burridge, P. W., Matsa, E., Shukla, P., Lin, Z. C., Churko, J. M., Ebert, A. D., Lan, F., Diecke, S., Huber, B., Mordwinkin, N. M., Plews, J. R., Abilez, O. J., Cui, B., Gold, J. D., & Wu, J. C. . Chemically defined generation of human cardiomyocytes. Nature methods, 11(8), 855-860, (2014)
Miyazaki, T., Futaki, S., Suemori, H., Taniguchi, Y., Yamada, M., Kawasaki, M., Hayashi, M., Kumagai, H., Nakatsuji, N., Sekiguchi, K., & Kawase, E. . Laminin E8 fragment support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nature communications, 3(1236), 1-10, (2012)
Taniguchi, Y., Ido, H., Sanzen, N., Hayashi, M., Sato-Nishiuchi, R., Futaki, S., & Sekiguchi, K. . The C-terminal region of laminin β chains modulates the integrin binding affinities of laminins. Journal of Biological Chemistry, 284(12), 7820-7831, (2009)
Ido, H., Nakamura, A., Kobayashi, R., Ito, S., Li, S., Futaki, S., & Sekiguchi, K. . The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin γ chains in integrin binding by laminins. Journal of Biological Chemistry, 282(15), 11144-11154, (2007)
FOR RESEARCH USE ONLY, NOT FOR USE IN DIAGNOSTIC PROCEDURES