STEM-CELLBANKER®
GMP grade
Preserving cells, tissues & organoids for regenerative medicine and cell therapy research
Product Basics
STEM-CELLBANKER® GMP grade is a chemically defined freezing solution with an optimized formulation for the storage of stem cells, iPS cells, and other valuable cells. Recent data also support its effectiveness for tissue cryopreservation. It is entirely free of serum and animal-derived components, containing only ingredients graded USP, EP, or JP. It’s a ready-to-use solution with a simple protocol that promises a safe and effective cryopreservation of stem cells and iPS cells.
STEM-CELLBANKER® GMP grade is manufactured in compliance with JPN, EU, US, and PIC/S GMP guidelines.
Key Features
- Chemically defined, free of serum and animal derived component
- Significantly increased cell viability while maintaining cell pluripotency, normal karyotype, and proliferation ability after freeze-thaw procedure
- Capable of cryopreserve tissue without damaging DNA or RNA
- Batch-to-batch stability
- Requires no further preparation; direct freezing at -80℃, with no programmable freezer required, etc.
- High quality product carefully monitored in compliance with GMP*
- Customizable**
* This product is U.S. FDA DMF registered. Contact us to request Letter of Authorization submission.
** For more information on customization, please click here.
Technical Information
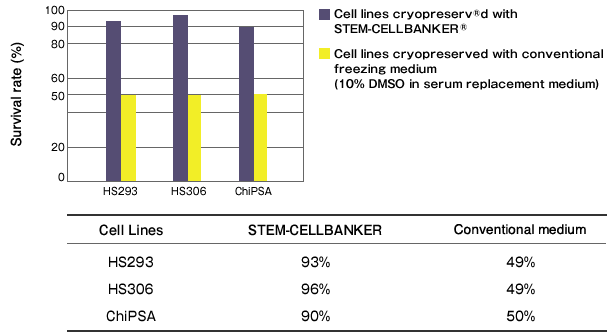
Cryopreservation analysis on hESC and iPS cells with STEM-CELLBANKER
By Department of Clinical Science, Intervention and Technology, Karolinska Institute
Tested cell lines: HS293 & HS306 (hESC); ChiPSA (human iPS)
Freezing medium: STEM-CELLBANKER® vs Conventional freezing medium (10% DMSO in serum replacement containing culture medium)
Cell viability assessment: trypan Blue & calcein-esterase based live-dead assay
Result: Significantly higher cell viability was observed while cell pluripotency, normal karyotype, and proliferation ability were maintained in HS293, HS306 and ChiPSA cell lines.
Special thanks to Prof. Outi Hovatta and Ms. Frida Holm for above analysis.Cryopreservation analysis on Mesenchymal Stem Cells (MSCs) and Endothelial Progenitor Cells (EPCs) with STEM-CELLBANKER®
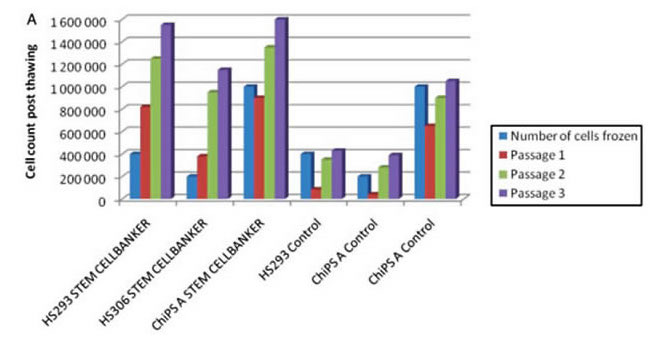
By Regenerative Medicine Faculty, University of Tsukuba
MSCs EPCs
STEM-CELLBANKER® >95% >90%
Tested cell lines: Human UCB-derived MSCs and EPCs
Freezing medium: STEM-CELLBANKER®
Freezing protocol: Cell froze directly at -80℃ for 3 days, transfer to -192℃ for 72 days.
Cell viability assessment: trypan Blue staining method.
Result: Cell viability as high as or more than 95% for MSCs and 90% for EPCs was observed while cell proliferation ability and differentiation ability were maintained in tested cells.
Special thanks to Prof. Osamu Ohneda and Dr. Masumi Nagano for above analysis.
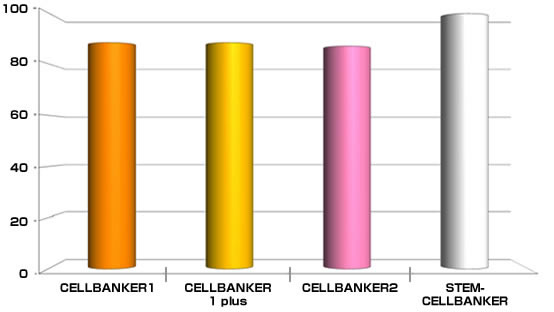
Cryopreservation analysis on Mouse ES cell (129SV)
By ZENOAQ cell engineering team
Tested cell line: 129SV (Mouse ES cell)
Freezing medium: CELLBANKER 1, 1plus, 2 and STEM-CELLBANKER®
Cryopresevation of mouse kidney tissue using STEM-CELLBANKER®
By ZENOAQ cell engineering team
Cryopreserved and thawed kidney tissue
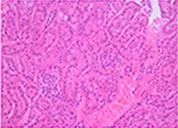
H&E staining
Active caspase 3 staining
Fresh kidney tissue
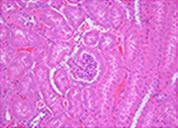
H&E staining
Active caspase 3 staining
Mouse kidney was minced into about 5 mm square. The tissue samples were either immediately fixed with 10% formalin or cryopreserved with 1 ml of STEM-CELLBANKER at -80°C for one week. The cryopreserved samples were fixed with 10% formalin immediately after thawing. Paraffin sections of both samples were stained with either H&E or anti-active caspase 3 antibody. Sign of necrosis or apoptosis was negligible in both fresh and cryopreserved tissue samples. Similar results were obtained from tissue samples from a variety of mouse organs.
Cryopreservation of mouse kidney tissue using STEM-CELLBANKER®
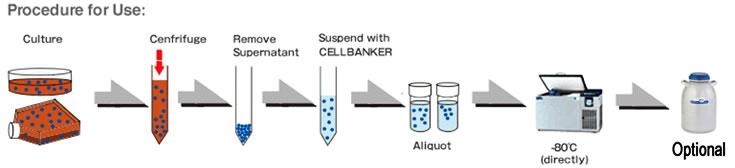
Protocol
Freezing
For optimum results, cells for cryopreservation should be in log phase of growth. Similar or other standard freezing protocols may be substituted.
- Examine and make sure the cell culture is free of contamination, in healthy and at proper confluency.
- Perform a cell count to determine the viability of cells.
- Centrifuge at 1,000 – 2,000 rpm, 4°C for 3 to 5 minutes to gently pellet the cells. Remove the supernatant with an aspirator.
- Gently suspend STEM-CELLBANKER® GMP grade cryopreservation medium (1 mL for 5×10⁵ – 5×10⁶ cells).
- Transfer 1 mL of the cell suspension to cryopreservation vial labeled with appropriate information (the cell line name, concentration, passage date etc.).
- Place the vials directly in -80℃ for storage.
- (OPTIONAL) Transfer the frozen vials to a liquid nitrogen storage tank after the vials have been frozen for at least 24 hours.
IMPORTANT: Optimum protocol may change with the cell types.
Thawing
- Remove the cryopreservation vial from the freezer and quickly thaw cells in a 37°C shaking water bath or shake by hand.
- Transfer the content to a centrifugation tube then immediately dilute and gently mix with 10mL of complete cell culture medium. Using CELLOTION® instead of complete culture medium will prevent adhesion of cells to the wall of the tube, increasing the recovery rate.
- Centrifuge cells at 1,000 – 2,000 rpm, 4°C for 3 to 5 minutes. Remove the supernatant with an aspirator.
- Gently resuspend the cells with an appropriate volume of complete cell culture medium then plate in a culture flask or plate
- Continue the culture procedures according to standard protocols.
Specification
- Manufactured By : Zenogen Pharma Co., Ltd
- Size : 20mL (SKU: 11922), 100mL (SKU: 11924) & 20mL x4 (SKU: 11922-S)
- Storage and Stability : 2 to 8℃ or below -20℃
- Expiration date : 3 years after the date of manufacture
- Disclaimer: STEM-CELLBANKER® GMP grade is not itself a pharmaceutical. Therefore, no warranty, express or implied, as to the fitness and suitability of this product for any particular purpose and/or merchantability unless the use is intended for research.
Pricing
STEM-CELLBANKER®
GMP grade
- Developed for stem cells
- iPS・ES cells cryopreservation
- Animal component free・Protein free
20ml size
SKU: 11922
Price: $60.00
100ml size
SKU: 11924
Price: $260.00
20ml size x 4
SKU: 11922-S
Price: $230.00
* This product is U.S. FDA DMF registered. Contact us to request Letter of Authorization submission.
References & Literature
Holm, F. et al. An effective serum- and xeno-free chemically defined freezing procedure for human embryonic and induced pluripotent stem cells. Human Reproduction 25, 1271–1279 (2010) doi: 10.1093/humrep/deq040.
Osaki, T., Uzel, S. G. M. & Kamm, R. D. On-chip 3D neuromuscular model for drug screening and precision medicine in neuromuscular disease. Nature Protocols 15, 421–449 (2020) doi: 10.1038/s41596-019-0248-1.
Doi, D. et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nature Communications 11, 3369 (2020) doi: 10.1038/s41467-020-17165-w.
Mae, S.-I. et al. Expansion of Human iPSC-Derived Ureteric Bud Organoids with Repeated Branching Potential. Cell Reports 32, (2020) doi: 10.1016/j.celrep.2020.107963.
Drummond, N. J. et al. Cryopreservation of midbrain dopaminergic neural cells differentiated from human embryonic stem cells. bioRxiv 2020.02.11.944272 (2020) doi: 10.1101/2020.02.11.944272.
Ballantyne, M. et al. Direct allele introgression into pure chicken breeds using Sire Dam Surrogate (SDS) mating. Nature Communications 12, 659 (2021) doi: 10.1038/s41467-020-20812-x.
Piao, J. et al. Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01. Cell Stem Cell 28, 217-229.e7 (2021) doi: 10.1016/j.stem.2021.01.004.
Tallapragada, N. P. et al. Inflation-collapse dynamics drive patterning and morphogenesis in intestinal organoids. Cell Stem Cell 28, 1516-1532.e14 (2021) doi: 10.1016/j.stem.2021.04.002.
Io, S. et al. Capturing human trophoblast development with naive pluripotent stem cells in vitro. Cell Stem Cell 28, 1023-1039.e13 (2021) doi: 10.1016/j.stem.2021.03.013.
Kondo, M. et al. Safety and efficacy of human juvenile chondrocyte-derived cell sheets for osteochondral defect treatment. npj Regenerative Medicine 6, 65 (2021) doi: 10.1038/s41536-021-00173-9.
Ishikura, Y. et al. In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 28, 2167-2179.e9 (2021) doi: 10.1016/j.stem.2021.08.005.
Vinel, C. et al. Comparative epigenetic analysis of tumour initiating cells and syngeneic EPSC-derived neural stem cells in glioblastoma. Nature Communications 12, 6130 (2021) doi: 10.1038/s41467-021-26297-6.
FOR RESEARCH USE ONLY, NOT FOR USE IN DIAGNOSTIC PROCEDURES